We will be building on the first year introductory extraction experiments ex. This technique can be used to separate covalent molecules from ionic compounds in an aqueous solution or suspension.
As part of your lab report include items 1 and 2 under the heading Experiment 4A on p.

. In most of the procedures summarised in these tables after homogenisation of the sample. It also has applications in the isolation of natural products as in the extraction of caffeine from tea leaves. 41 of the textbook.
In solvent extraction a distribution ratio is often quoted as a measure of how well-extracted a species is. The selectivity of the extraction experiment involving pH manipulation can be further improved using a technique known as Back Extraction. Answer these quiz based flashcards based on the Liquid Liquid Extraction Theory Questions and check your knowledge.
Depending on the system the distribution ratio can be a function of temperature the concentration of chemical species in the system and a large number of other parameters. Liquidliquid extraction LLE is based on the principle that a solute or an analyte can distribute itself in a certain ratio between two immiscible solvents usually water aqueous phase and organic solvent organic phase. The distribution ratio Kd is equal to the concentration of a solute in the organic phase divided by its concentration in the aqueous phase.
Extraction is a fundamental technique used to isolate one compound from a mixture. Draw a flow diagram similar to that in Figure 610 for the substances 24 and 6 shown in Figure 615. Also include also items 1 and 2 under the heading.
Fortunately observing such a procedure allows to scale up from the beaker to the. In a typical LLE experiment an aqueous sample is mixed with an apolar nonmiscible solvent like n-hexane. 1 - 1 Experiment 1.
Add drying agent until the organic solvent is sufficiently dried of aqueous solvent. LiquidLiquid Extraction PreLab. Where ionic species are removed from a non-polar solvent by extraction into water.
ABSTRACT SUMMARY In liquid-liquid extraction experiment it consists of two parts. Where a solid or liquid suspended or dissolved in one solvent is extracted into another. Liquid-Liquid Extraction This experiment serves as a general introduction to the Chem 235 Organic Chemistry Laboratories.
Note that D is related to the ΔG of the extraction process. Observation were made during the first extraction of each liquid from the solution after the extraction and during recrystallization and purification. Isolation of Caffeine from your first year chemistry labsYou will be separating a mixture of two compounds triphenylmethanol a neutral organic compound and.
The extraction technique can be used to purify compounds or to separate mixtures of compounds such as when isolating a product from a reaction mixture known as an extractive work-up. Introduction Extraction is the drawing or pulling out of something from something else. Drying the Organic Solvent.
Once the target analytes are in the organic phase they can be re-extracted into a fresh aqueous phase whose pH has been manipulated to ensure the analytes are in the charged form and therefore most highly. Liquidliquid liquidsolid and acidbase also known as a chemically active extraction. If the drying agent forms a clump at the bottom of the tube then more drying agent is needed.
A schematic diagram of a complete liquid-liquid extraction process from Separation Process Engineering by Wankat 2007 In the extraction process the feed which contains the first solvent or the diluent and the solute is. Liquid-liquid extraction is the separation method preferred by most of the authors for the first step in the isolation of the analytes from the food matrix. The technique of liquid-liquid extraction is used to purify impure substances by taking advantage of a solubility differential of the substance in different.
The three most common types of extractions are. In Tables 51 and 52 analytical studies published since the 1999 review are described. Throughout this experiment the desired goals to be accomplished are to analyse the extraction of a part from a binary liquid mixture by a solvent using a liquid-liquid extraction process then calculate its percentage recovery in a continuous operating mode.
NC State University Organic Chemistry Lab Introduction to basic organic laboratory equipment and techniqueshttpwwwncsueduchemistry. Prepare a PreLab as you have for the last two experiments and do this exercise. Firstly to determine the distribution coefficient for the system organic solvent-Propionic acid-water and to show it dependence on concentration and the second is to demonstrate how a mass balance is performed on the extraction column and to measure the mass.
Sample Calculations 3-Discussion of Results 4-Appendices Appendix A Figures 5-. Department of Chemical Engineering Illinois Institute of Technology. Becoming familiar with its theory and correct use are essential to successful completion of many organic experiments.
Learn everything related to the Liquid Liquid Extraction Theory with the help of our flashcards quizzes with ease. Table 1 shows the observations made at various points in the experiment for each compound being separated using liquid-liquid extraction. A drying agent such as magnesium sulfate can be used to further extract aqueous solvent from the organic solvent after extraction.
Home Create Flashcards Science Experiment Can You Answer Following Questions On Liquid. Results and Calculations This experiment focused on the technique liquid-liquid extraction.

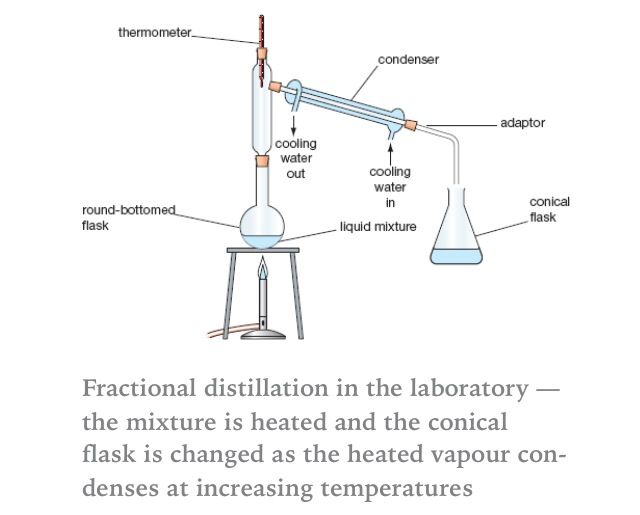
Fractional Distillation Fractional Distillation Chemistry Organic Chemistry

Blog Guest Hollow Science Experiments High School Science Science Fair

Extracting Dna From A Strawberry Biology Experiments Science For Kids Dna

This Is The Flottweg Two Phase Decanter The Liquid Is Discharged By Gravity It Is Used For Processing Drilling Decanter Modular Design Sewage Treatment Plant

A Comprehensive Guide To Essential Oil Extraction Methods Essential Oil Perfume Essential Oil Extraction Making Essential Oils

In This Science Lab You Extract And Isolate Dna From Strawberries Using Simple Household Ingr Science Experiments Life Science Experiments Elementary Science

Distillation Making Essential Oils Essential Oil Distiller Oils

A Chemical Engineer S Guide Of Solid Liquid Extraction Chemo Concept Nursing Student Tips Nerd Jokes Chemo

Essential Oil Steam Distillation Kit Lab Apparatus W Hot Stove Graham Condenser Deschem Essential Oil Distiller Making Essential Oils Distillation

Everywhere Where Liquids Have To Be Clarified Or Fine Solids Have To Be Separated From A Liquid Clarifying Disc Stack Centrifuges Separators Centrifuges Stack

Pin On Experimenten En Veiligheid

Pictures From An Organic Chemistry Laboratory Soxhlet Extractor Organic Chemistry Glass Stopper

Strawberry Dna Extraction Lab For Kids Little Bins For Little Hands Science Activities For Kids Dna Extraction Lab Dna Activities

Solvent Extraction General Making Essential Oils Distillation Oils